PCR Bizarre
COVID-1984 and its "gold standard"
All the COVID measures taken by the authorities, including extremely destructive global lockdowns and isolation of countless people, were largely based on PCR testing. The RT-PCR test is presented to the world as the so-called "gold standard" for testing if the specific COVID-19 virus is present in a person although this is questionable. The goal of testing is to know whether a person is infectious in order to prevent the spread of the virus.
PCR in a nutshell... The viral genetic RNA material is reverse transcribed into cDNA, unique primers* demarcate the specific sequence which is then amplified multiple times using a reaction catalyzed by an enzyme, a proces called enzymatic DNA synthesis (in vitro). With each amplification a DNA helix is separated and sequences from the enzyme attach to these separated strands at the right locations to form two new but identical DNA helixes. Each amplification is called a "cycle of amplification". This results in an exponential curve of amplification*.
The Cycle threshold (Ct)
The amplification "Cycle threshold" (Ct) value correlates with viral load. A lower Ct value implies a higher viral load in the sample because the virus was already detected after fewer cycles, and vice versa. At start of infection of a person the viral load is low while a few days after infection it peaks and after 10 to 15 days the virus is no longer infectious.*
However, in practice Ct values are not used to determine viral load in people because of many complicating factors that may influence the result. The PCR test is very sensitive and the specimen is prone to contamination. Above a certain threshold called the "Ct cutoff value" the test will practically always give a false positive. Below this Ct cutoff value a positive is most likely a true positive when done correctly. This was admitted in July 2020 by Anthony Fauci:if you get a cycle treshold of 35 or more that the chances of it being replication competent are minisculeand if the treshold is above that value thenit's just dead nucleotides. Period.*
It can be observed that at Ct = 25, up to 70% of patients remain positive in culture and that at Ct = 30 this value drops to 20%. At Ct = 35, the value we used to report a positive result for PCR, 3% of cultures are positive.
That means that if someone is tested by PCR as positive when a threshold of 35 cycles or higher is used (as is the case in most laboratories in Europe & the US), the probability that said person is actually infected is less than 3%, the probability that said result is a false positive is 97%.
A cycle threshold cut-off 33-35 was associated with low infectivity. Clinical laboratories often use Ct cut-off provided by the RT-PCR kit manufacturers which are between 37 and 40 cycles. ... Our results show that the 97.5th percentiles of the lower peaks (ORF1ab and N) which is (~32 cycles) may represent the maximum diagnostic efficiency cut-off.
Laboratories around the world, especially in the western world, used a threshold of 35 or higher and therefore likely many false positives were detected which of course kept the number of COVID-19 infections artificially high. These high tresholds were "advised" by organizations like CDC, FDA and WHO. The inflated numbers headlined in the media and were used to justify destructive measures like lockdowns and quarantaines.
Wanted: Dead or Alive
During the Coronavirus Disease 2019 (COVID-19) pandemic, residual SARS-CoV-2 genome and subgenomic RNA fragments were observed in recovered COVID-19 patients. The presence of such RNAs in the absence of live virus leads to incorrectly positive RT-qPCR results, potentially delaying medical procedures and quarantine release. We here propose a simple modification to turn commercial COVID-19 RT-qPCR protocols into long-range RT-qPCR assays that can differentiate between infectious and non-infectious influenza and coronavirus RNA levels.
It was already known before the COVID-19 crisis that a PCR test cannot determine whether a detected virus segment is part of a live infectious virus or dead virus particles.
Even the most fundamental physiological state, viability, cannot be assessed cross-sectionally by standard DNA-targeted methods such as PCR.
When a vaccinated cell dies or is destroyed by the immune system, the debris may release a large amount of Spike proteins and protein fragments. So a PCR may well detect RNA residues of a past infection while the person in question is not infected and cannot spread the virus. In an FDA guidebook for PCR testing of COVID-19 we read under the header limitations :Detection of viral RNA may not indicate the presence of infectious virus or that 2019-nCoV is the causative agent for clinical symptoms.
This might also explain to some extent why so many people were labeled "asymptomatic", they tested positive but showed no symptoms. So while in the beginning of the "pandemic" the authorities and their media tried to make the world believe that people could get reinfected with COVID-19 after initial infection it was already widely known and reported that dead virus fragments are causing COVID-19 reinfection false positives.*
screenshot
We know that most transmissions occur at a very early stage of the disease, during the first 5 days after onset of symptoms. But for example in one study more than 50% of COVID-19 patients tested positive again 21–25 days after their initial positive test. In another study the longest duration of viral RNA shedding could be 83 days using PCR procedures as recommended by CDC and WHO. So that means that PCR detectable dead virus parts remain in the system long after the infection is gone. Knowing now that PCR tests were not designed to prevent false positives we can safely state that many reinfections are either caused by virus variants or by false positives from increased PCR testing around the world. For example in January 2022 The Guardian reported about reinfections* but did not mention false positives as a possible cause anywhere in its article. Just like the CDC in its article. That is either by ignorance or by omission.
PCR test kit
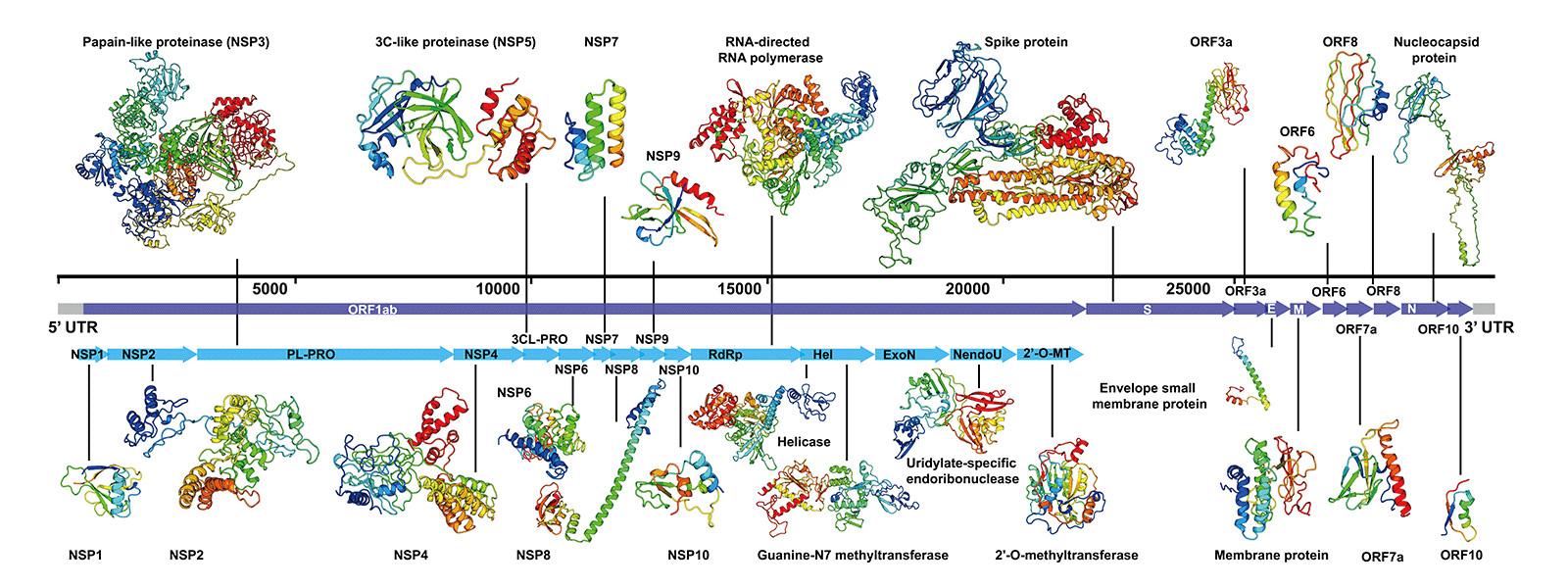
It is of course crucial to know which unique genetic virus strain is exactly being tested and amplified. Different corona viruses can be genetically similar and share much of the genetic code with each other. If parts of the genetic code are being amplified which are also part of other viruses then the test is more likely to give positive results, even when there's no SARS-CoV-2 virus present. Let's check an example of a PCR test kit..
According to the instructions of an Astra BioTech PCR kit...
SARS-CoV-2 Triplex PCR kit simultaneously detects three targets, in ORF1ab, ORF8, and N protein coding regions, each on separate detection channel. The detection of 3 regions increases the specificity of the test and makes it stable against potential mutations of the virus in the regions where primers or probes bind.*
This PCR kit tests for so-called targets ORF1ab, ORF8, and N protein coding regions. ORF1ab is a part of the genome of nidoviruses which include corona viruses. ORF8 is a common gene in betacoronaviruses and one of the most variable parts of the genome. N protein is a common protein in coronaviruses. These are being sold as "SARS-CoV-2 specific regions", but they don't specifically target for SARS-CoV-2 RNA, but for coronaviruses in general.
Isolation of SARS-CoV-2
For a SARS-CoV-2 test to be meaningful the unique SARS-CoV-2 virus must have been isolated in a lab and its exact unique genetic code must be known in order to include specific and unique targets in the test design. But there's some controversy surrounding the isolation of SARS-CoV-2. Skeptics claim that an infectious agent must be completely isolated and purified, injected into a healthy animal or human and shown to cause the same disease, according to Koch's postulates. This may be possible for bacteria because they survive without a host, but viruses can't survive without a living host like a cell. Koch's postulates date to the 19th century but science and technology advanced much since then. By means of electron-microscopy viruses can be observed* and their unique shapes and characteristics can be determined* and confirmed by independent observations around the world.
DNA or RNA viruses can be isolated by means of for example ultrafiltration, ultracentrifugation, chromatography and nano magnetic bead technology, but it's time consuming and the isolated and especially the purified virus RNA is very vulnerable. Viruses are usually cultivated in cell culture, an infection results in cell lysis and formation of a viral plaque while non-infected cell cultures remain unaffected and healthy. The culture is then put in a living host to see the effect. This is done with control experiments which show the difference in effect on the specimen with or without infected or non-infected culture.
Despite the controversies surrounding certain viruses, like for example the HIV controversy, there are certainly viruses that cause infection. For example the Ebola virus is always present and unique in Ebola, not in other illnesses* and influenza has been around at least as long as humanity itself.
The first full SARS-CoV-2 genome that supposedly was sequenced was China's Wuhan isolate, GenBank: MN908947.3*. It was published in January 2020. Soon the USA isolate followed GenBank: MN985325.1*. It was published beginning of 2020. On 12 July 2020 the FDA made the following statement...
During the early months of the Coronavirus Disease 2019 (COVID-19) pandemic, clinical specimens were not readily available to developers of IVDs [In Vitro Diagnostics] to detect SARS-CoV-2. Therefore, the FDA authorized IVDs based on available data from contrived samples generated from a range of SARS-CoV-2 material sources (for example, gene specific RNA, synthetic RNA, or whole genome viral RNA) for analytical and clinical performance evaluation. While validation using these contrived specimens provided a measure of confidence in test performance at the beginning of the pandemic, it is not feasible to precisely compare the performance of various tests that used contrived specimens because each test validated performance using samples derived from different gene specific, synthetic, or genomic nucleic acid sources.*
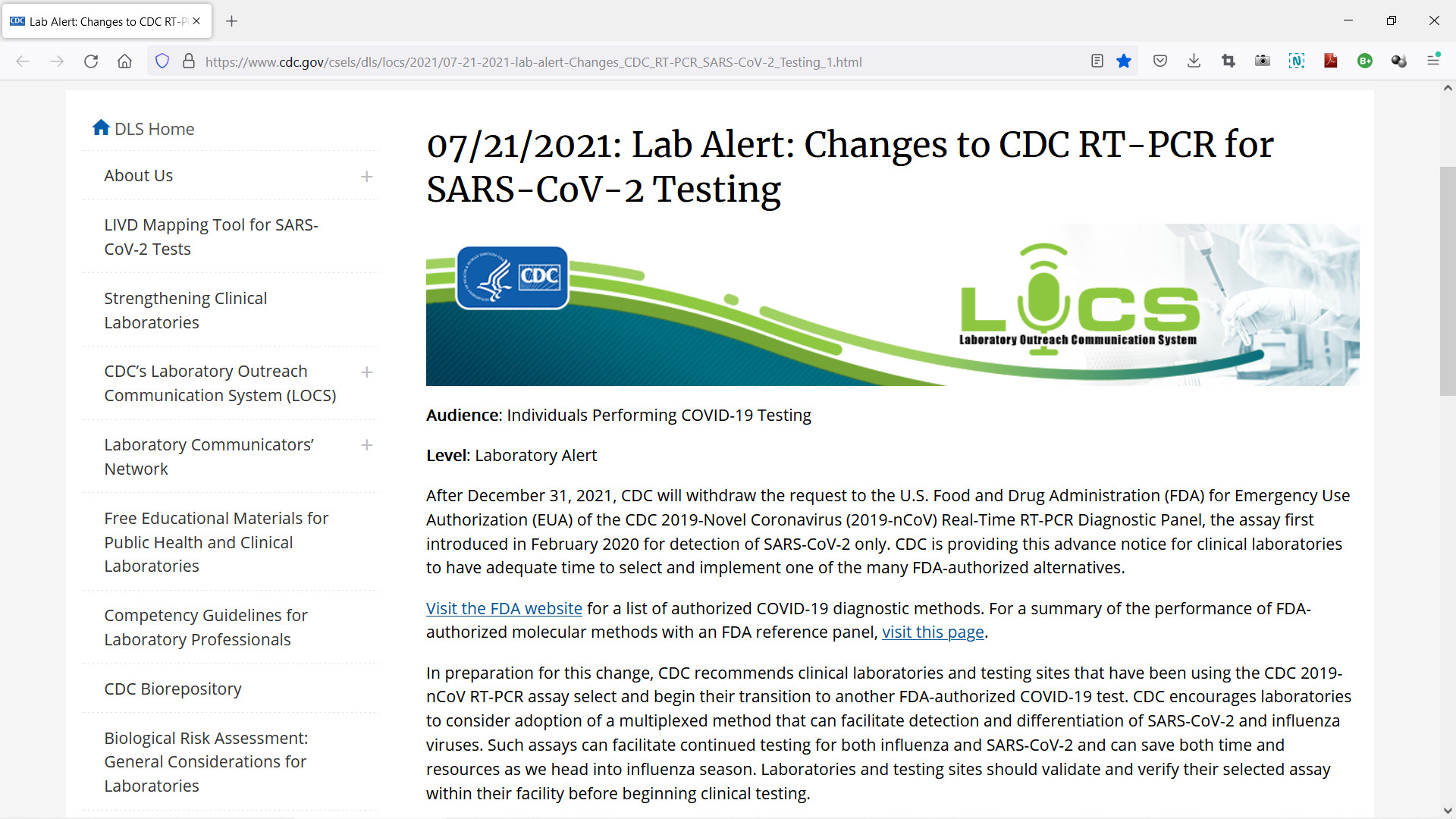
Despite there being an isolate of the SARS-CoV-2 virus genome, the FDA here states that in the beginning of the so-called pandemic "clinical specimens were not readily available". Does this mean that the isolate which was published at the beginning of 2020 was not 100% reliable? On 19 July 2021 the CDC retracted its PCR test protocol...
After December 31, 2021, CDC will withdraw the request to the U.S. Food and Drug Administration (FDA) for Emergency Use Authorization (EUA) of the CDC 2019-Novel Coronavirus (2019-nCoV) Real-Time RT-PCR Diagnostic Panel, the assay first introduced in February 2020 for detection of SARS-CoV-2 only. CDC is providing this advance notice for clinical laboratories to have adequate time to select and implement one of the many FDA-authorized alternatives. ... CDC encourages laboratories to consider adoption of a multiplexed method that can facilitate detection and differentiation of SARS-CoV-2 and influenza viruses. Such assays can facilitate continued testing for both influenza and SARS-CoV-2 and can save both time and resources as we head into influenza season.*
Only at the end of 2021, the year of mass vaccinations, the CDC withdrew the RT-PCR assay which were implemented in the beginning of 2020 and which supposedly tested specifically for COVID-19. But a few days earlier FDA announced that the isolate used to determine the PCR test was not 100% reliable. And therefore not reliable to test for COVID-19 specifically? Interestingly, in 2020 it seemed as if the yearly flu outbreak didn't take place, because all people with flu-like symptoms were labeled COVID-19 patients, largely based on these (unreliable) PCR tests. By the end of 2021 the CDC started advising to test for COVID-19 and influenza or common flu...
Symptoms for COVID-19 and the Flu can be similar, so testing for all three viruses at the same time will provide public health officials with information they need to help reduce the spread of these viruses in the community while conserving resources that are in short supply.**
On 21 July 2021 the FDA made the following statement...
The agent detected may not be the definite cause of disease. ... Since no quantified virus isolates of the 2019-nCoV were available for CDC use at the time the test was developed and this study conducted, assays designed for detection of the 2019-nCoV RNA were tested with characterized stocks of in vitro transcribed full length RNA (N gene; GenBank accession: MN908947.2) of known titer (RNA copies/μL) spiked into a diluent consisting of a suspension of human A549 cells and viral transport medium (VTM) to mimic clinical specimen.
So at the time the first test was developed "no quantified virus isolates of the 2019-nCoV were available". It was based on the first Wuhan genome GenBank: MN908947.2 of January 2020 which was a precursor of the improved GenBank: MN908947.3 which was released a few days later. So the test in use for such a long time, until end of 2021, was not even based on the latest Wuhan genome version and apparently also not on the USA genome because it is not mentioned. Then in November 2021 a paper was published which claimed that the specific COVID-19 virus still hadn't been properly isolated in a lab...
We find that PCR testing data for the second and following waves of COVID-19 pandemic indicate that these waves are mainly artefacts of false-positive results. We find that this interpretation provides a more consistent explanation of the known epidemiology of COVID-19 than the hitherto consensus notion of extremely contagious and rapidly mutating viruses. ... Consulting the respective seminal publications, we find that the respective genetic material had been identified computationally without preparing an isolate of the respective virus particles, and without separating them physically from other carriers of genetic material that may be present in the biological samples. Noting that tests apparently produce false positive results in people carrying some respiratory virus different from SARS-CoV-2, we must conclude that the alleged genetic code of the SARS-CoV-2 virus had been wrongly identified...
The SARS-CoV-2 virus was only identified "computationally" while other genetic material might have been "present in the biological samples". The authors believe that the reason for so many false positives is because the COVID-19 virus was wrongly identified to start with and included other corona or flu virus sequences as well. This might explain to some extent why the flu seemingly "disappeared" in 2020 and how the authorities were able to report exaggerated COVID-19 cases and deaths worldwide in order to get support for their destructive lockdowns and measures. Everybody with the yearly common flu was labeled COVID patient. See also COVID-1984.
Kary Mullis
source
Kary Mullis won the Nobel Prize in Chemistry in 1993 for inventing the PCR test. He confirmed that the result of a PCR test does not tell whether you're sick or not or whether you're infectious or not because it also detects dead virus parts that are left over from previous infection. It would have been extremely interesting to know what a true scientist like him would think about the whole COVID-1984 episode, especially the PCR debacle which is being misused to support incredibly destructive global lockdowns and massive power shifts from people to power elite. In May of 1996, Mullis was interviewed by Dr. Gary Null in which he talked about HIV and Dr Fauci, the director of the National Institute of Allergy and Infectious Diseases, or NIAID, and the Chief Medical Advisor to the US President, who played a leading role in the COVID-1984 pLandemic. He said the following...
source
Those guys have got an agenda which is not what we would like them to have, being that we pay for them to take care of our health in some way.
See also UNhealth. Unfortunately Mullis died on August 7, 2019, shortly before the outbreak of COVID-19, from complications of pneumonia. How bizarre. Related to HIV PCR testing Mullis once stated...
Quantitative PCR is an oxymoron. ... PCR is intended to identify substances qualitatively, but by its very nature is unsuited for estimating numbers. Although there is a common misimpression that the viral-load tests actually count the number of viruses in the blood, these tests cannot detect free, infectious viruses at all; they can only detect proteins that are believed, in some cases wrongly, to be unique to HIV. The tests can detect genetic sequences of viruses, but not viruses themselves.